1 Global climate and the greenhouse effect
1.3 Energy flows within the Earth-atmosphere system
Before we focus on the enhanced greenhouse effect, we need to refine the schematic representation in Figure 7 and draw in some of the other processes that influence the Earth's temperature – not only at the surface, but also at different levels within the atmosphere.
1.3.1 The vertical ‘structure’ of the atmosphere
The atmosphere is not a simple, uniform slab of absorbing material. On the contrary, it gets progressively ‘thinner’ or less dense with increasing altitude (height above mean sea level); i.e. the total number of molecules in a given volume of air is lower, and so is the pressure. About 80% of the total mass of the atmosphere is within some 10 km of the surface; 99.9% lies below 50 km.
The important corollary is that the key greenhouse gas molecules (H2O and CO2) are also more abundant close to ground level, and increasingly scarce at higher altitudes. So a better picture of radiation trapping in the real atmosphere is to imagine it happening in a series of stages. Outgoing longwave radiation is repeatedly absorbed and re-emitted as it ‘works up’ through the atmosphere; it is re-radiated to space only from levels high enough (i.e. thin enough) for absorption to have become weak. This suggests that the atmosphere should be warmer at ground level – close to the source of the outgoing radiation, and where the absorbing molecules are more abundant. Everyday experience confirms this expectation; it generally gets colder as you walk up a mountain, for example.
Figure 9 is a typical temperature profile of the atmosphere. It shows that air temperature does indeed fall with increasing altitude throughout the lower atmosphere or troposphere, reaching a minimum value (of about −55 °C) at the tropopause. This lies 8–15 km above the ground, depending mainly on latitude: it is higher (and colder) at the Equator than at the poles. No mountains rise above the troposphere; it is where we live and where almost all weather phenomena (rain, clouds, winds, etc.) occur. However, if you could travel higher up (without the protection of a jet aircraft), you would find that the temperature soon starts to increase again – and continues to do so up to the stratopause at the top of the stratosphere. Why is this?
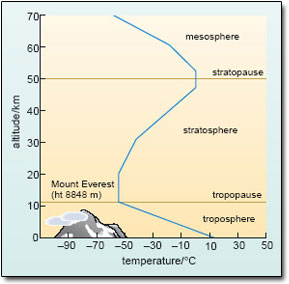
Figure 9: The characteristic temperature profile of the atmosphere produces a vertical structure like a series of concentric shells. The successive regions or ‘spheres’ are separated by ‘pauses’ where the change in temperature with altitude switches from decreasing to increasing, or vice versa. The outer more-rarefied reaches of the atmosphere (which extends up to 100 km or so) are not included.
1.3.2 The fate of incoming solar radiation
Look back at Figure 7. In this schematic representation, what is the fate of incoming solar radiation?
It is either reflected back to space (31 units) or absorbed by the surface (69 units).
Some solar radiation is, in fact, absorbed as it travels down through the atmosphere. Mostly, this is radiation at wavelengths in the two ‘tails’ of the solar spectrum (Figure 5) – the ultraviolet and the near infrared.
Like water vapour and CO2, the ozone in the troposphere acts as a greenhouse gas. Unlike those two gases, however, very little of the Earth's ozone is, in fact, in the lower atmosphere; the bulk of it (some 90%) is in the stratosphere, where it forms the so-called ozone layer. In this more-rarefied region, ozone plays a different role because it also absorbs the shorter ultraviolet wavelengths in the solar spectrum – radiation that is lethal to many micro-organisms and can damage important biological molecules, leading to conditions such as skin cancer in humans. Fortunately for life on Earth, most of this radiation is absorbed by the ozone layer, preventing it from penetrating deeper into the atmosphere. More pertinent here, the absorption of incoming solar energy by stratospheric ozone heats this region of the atmosphere directly. In effect, the stratosphere is heated from above, whereas the troposphere is heated from below. This is why the highest temperatures are found at the top of the stratosphere, but at the bottom of the troposphere (as shown in Figure 9).
About half of the incoming near-infrared radiation is also absorbed, mainly by water vapour low down in the troposphere. In addition, the atmosphere contains a huge assortment of aerosols – fine solid particles and liquid droplets suspended in the air. Except in the aftermath of a major volcanic eruption (of which more in Section 1.5), aerosols are also most abundant in the lower atmosphere; natural sources include desert dust wafted into the air by wind, smoke and soot from wildfires, salt from sea-spray, and so on. Depending on their make-up, aerosols can absorb solar radiation – or (and this is usually more important) scatter some of it back to space. Globally, aerosols make a significant contribution to the Earth's albedo (included in the figure of 31% quoted earlier). They also play another important role. Many aerosols act as cloud condensation nuclei, providing surfaces that promote the condensation of water vapour to form the liquid droplets (or ice crystals, at higher and colder altitudes) suspended in clouds – a process that occurs less readily in ‘clean’ (i.e. aerosol-free) air.
1.3.3 The role of clouds
We have already identified one role that clouds play in the Earth's climate: they are highly reflective (Section 1.2.1). At any given time, about half of our planet is covered by clouds; the sunlight they reflect back to space accounts for about 55% of the total planetary albedo. However, clouds also absorb and re-emit outgoing longwave radiation; i.e. they contribute to the back radiation from the atmosphere, and hence to the natural greenhouse effect. This is why temperatures tend to be lower under clear night skies than on nights with extensive cloud cover.
Thus, clouds present something of a paradox: they both warm and cool the Earth. The balance between these two opposing effects is a delicate one – dependent on factors such as the type and thickness of the clouds, their altitude, whether they consist of water droplets or ice crystals, and so on (Figure 10). Averaged over time and around the world, satellite data indicate that the net effect of clouds in our current climate is a slight cooling of the surface. As you will see, predicting how the balance between warming and cooling might shift in a warmer world remains one of the biggest headaches for climate scientists.
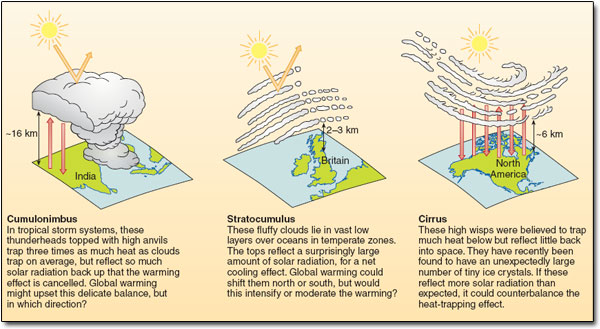
Figure 10: Researchers are only beginning to understand the complex role clouds play in modulating the planet's temperature. The figure summarises some key points, stressing how different types of clouds affect the Earth's radiation balance differently. How these variations fit together to produce a global cooling effect, and how that might change in a warmer world, remains uncertain.
1.3.4 The role of convection in the atmosphere
We come now to our final refinement to the simple picture in Figure 7. Recall that the troposphere is heated from below, with temperature then falling with increasing altitude. This situation sets the scene for the onset of convection – the bulk flow or circulation of a fluid driven by differences in temperature. Convection in the atmosphere plays a vital role in two further mechanisms – quite apart from the emission of longwave radiation – whereby energy is transferred from the Earth's surface to the atmosphere.
The first is the transfer of ‘thermal’ energy (often referred to rather loosely as ‘heat’) by a combination of conduction and convection. This is essentially the same mechanism that heats a saucepan of water on the stove; see Box 4. The situation in the atmosphere is more complicated, but the basic principle is the same. Warm air, heated by contact with the ground or a warm sea, rises upwards carrying heat transferred from the surface aloft. This allows more cool air to come into contact with the surface and be heated in its turn. Working together, conduction/convection drive a significant flow of heat across the boundary between the surface and the air.
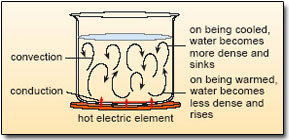
Box 4 Heating water by conduction and convection
Anyone who tries to pick up a metal spoon left in contact with a hot pan quickly learns that metals are good conductors of heat. Conduction is the transfer of heat through matter by molecular activity; i.e. the energy is transferred through contact between individual molecules. By contrast, convection is the transfer of heat by bulk movement or circulation within a fluid (a liquid like water or a gas like the air).
In Figure 11, heat is transmitted from the electric element, through the pan to the water in contact with the base of the pan by conduction. As water in this layer warms up, it expands – this is called thermal expansion – and so becomes less dense than the water above. Because of this new buoyancy, the warm water begins to rise, to be replaced by cooler, denser water from above which is heated in its turn. On reaching the surface, the warmed water begins to lose heat to the air; it cools, becomes denser and sinks, then is heated again and rises, and so on. As long as the water is heated unequally (i.e. from the bottom up), the water will continue to ‘turn over’ in a convective circulation so that eventually all of it becomes warm.
The second form of energy transfer is indirect, but even more important on a global scale. It involves the evaporation of water – mainly from the oceans, but also from lakes and rivers, soils, rocks and vegetation on land. Evaporation requires energy, known as the latent heat of vaporisation, which is extracted from the surface involved. This is why the evaporation of sweat acts to cool the body. The latent heat of vaporisation of water, i.e. the amount of heat needed to convert 1 kg of liquid water to water vapour at the same temperature (and the amount of heat released to the surrounding environment when 1 kg of water vapour condenses) is 2.25 × 106 J kg−1 – higher than the value for any other substance.
How does convection in the overlying air help to promote the evaporation of water?
Convection carries air containing water vapour upwards, so the air just above the surface does not become ‘saturated’ (Section 1.2.2), enabling more water to evaporate.
As we noted earlier, the saturation limit of air depends on temperature: cool air can carry less water vapour than warm air. As moisture-laden air is carried upwards, it cools and may become saturated. Continued rise and further cooling then results in the condensation of water vapour onto aerosols in the air: clouds form and latent heat is released to the atmosphere. Clouds, the turbulence of atmospheric convection and the winds that redistribute heat around the world are largely confined to the troposphere (tropos is Greek for ‘turning’).
Look back at Figure 9. It is often said that the tropopause acts like a lid, preventing convection in the lower atmosphere from reaching any higher. Can you suggest why?
With (less dense) warm air lying above (more dense) cooler air, conditions in the stratosphere are not conducive to convection. (Stratos is Latin for ‘layered’.)
Rapidly rising air can (and does) overshoot the tropopause, mostly in the updraught of violent storms over the tropics. And there are return routes as well, mainly at middle latitudes. In general, though, the circulation of air in the stratosphere does not interact strongly with the wind systems in the lower atmosphere. It is within the troposphere that the full drama of the Earth's weather occurs.